Innovative solutions for life science challenges
WHAT WE DO
It takes rigorous science, innovative strategies, and world-class expertise to tackle difficult life science issues and keep your projects moving forward. That’s what BlueRidge Life Sciences provides.
We offer integrated, comprehensive solutions that address complex technical, clinical development, and safety and regulatory challenges, leveraging our deep scientific, medical, and regulatory expertise and real-world experience. Our goal is to help you complete projects efficiently and with less risk, leading to solutions for a healthier and safer world.
With a broad range of renowned scientific, medical, regulatory, and engineering specialists under one roof, we streamline your project and reduce your workload, so you can make sound, science-based decisions about human and environmental health issues.
BlueRidge Life Sciences is a growing family of companies providing a comprehensive suite of life sciences services, spanning toxicology, regulatory science, risk assessment, epidemiology, biostatistics, engineering, clinical research, health economics, pharmaceutical commercialization, and environmental consulting. Across these specialized areas, we collaborate to deliver science-driven solutions that support industries including pharmaceuticals, biotechnology, medical devices, chemicals, foods and consumer products. Our collective expertise helps clients navigate regulatory requirements, assess safety and efficacy, manage risks, and optimize pharmaceutical commercialization strategies.
If you’re ready to achieve your life science goals, there’s no better partner than BlueRidge Life Sciences.
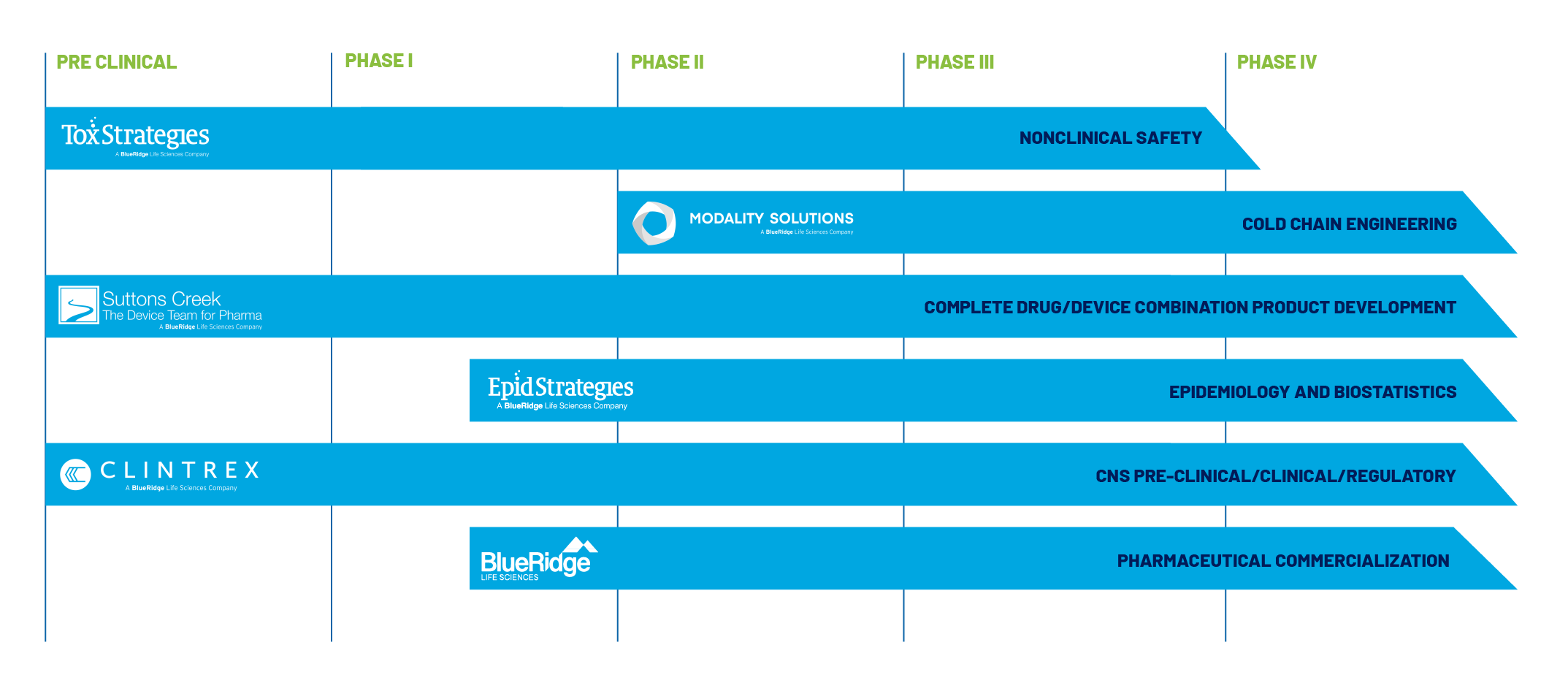
WHAT WE DO
For therapeutic product development, we offer a cross-division integration of expertise to support pharmaceuticals, biologics, and medical device development from discovery through commercialization.
Clintrex Research provides strategic guidance on CNS therapies from pre-clinical trial design through regulatory submissions, and investor strategies. EpidStrategies uses rigorous methodologies for evidence planning, integration, and synthesis to support and advance drug and medical device development, treatment, and safety. HEORStrategies and BlueRidge Pharmaceutical Commercialization delivers health economics and outcomes research (HEOR) to support value demonstration, pricing strategies, and reimbursement planning and to define the value of therapies for payers, patients and providers. Modality Solutions ensures the integrity of temperature-sensitive therapeutics through engineering solutions for cold chain logistics. Suttons Creek Inc. plays a key role as the device team for pharma, offering specialized consulting in medical device development, combination products, and regulatory strategy. Our expertise helps ensure that drug-delivery systems, diagnostics, and device-based therapies meet rigorous industry standards, regulatory requirements and ultimately reach the patients who need them.
ToxStrategies provides a comprehensive range of services in nonclinical toxicology for clinical development of biopharmaceutical (innovator and biosimilars) and pharmaceutical drug candidates. Together, we provide an integrated, end-to-end approach to life sciences consulting, supporting clients in developing, validating, and bringing therapeutic innovations to market efficiently and safely.
Technical Services Across the Therapeutic Product Development Lifecycle
BlueRidge supports the most challenging phases of drug development, including nonclinical safety, clinical development, regulatory strategy and commercialization. We have solved some of the most difficult challenges during the pIND, IND, EOP2, Pre-NDA and NDA phases of development.
Our world-renowned team of physicians, scientists, engineers, health economists, and pricing and pharmaceutical commercialization experts brings deep expertise in both traditional medications and innovative therapeutics—including combination drug/biologic + device products, cell and gene therapies, monoclonal antibodies, and small-molecule drugs. BlueRidge leverages experience across a full spectrum of therapeutic areas, including central nervous system (CNS) disorders and pain, rare diseases, infectious diseases, oncology, ophthalmology, and autoimmune and inflammatory conditions. Additionally, we utilize advanced simulation methodologies to perform transport validation testing on novel, fragile, or temperature-sensitive therapeutics, ensuring they meet rigorous regulatory requirements during transit.
Epidemiology and Biostatistics
BlueRidge epidemiologists and biostatisticians provide innovative and robust solutions for conducting, evaluating, and interpreting epidemiological studies in the pharmaceutical, medical device, nutritional product, and environmental chemical industries. Our research frequently results in peer-reviewed publications and presentations at scientific conferences and is also used in numerous regulatory documents in the US and Europe.
Our scientists are well versed in evidence generation, real world data and evidence (RWD/RWE), value propositions, study design and protocol development, statistical analyses, regulatory submissions, and publication strategies.
Exposure Sciences
Our scientists, engineers, and health physicists are recognized leaders in assessing exposure to chemicals and radionuclides in air, water, soil, sediments, and biota, as well as in food and consumer products. In addition, we provide customized industrial hygiene and occupational safety services.
BlueRidge has extensive experience in designing and implementing exposure monitoring studies, compiling and analyzing large environmental datasets, and collaborating with federal, state, and local regulators within regulatory programs such as Superfund and TSCA. We leverage state-of-the-art tools to perform quantitative modeling and apply GIS methods to assess and visualize exposure risks from air toxics and other environmental releases. Our expertise also includes exposure reconstruction, exposure simulation studies, and research on bioaccessibility and bioavailability.
Risk Assessment, Toxicology, and Health Science Research
BlueRidge toxicologists, scientists, and engineers bring decades of experience and PhD-level expertise in designing research programs and conducting risk assessments for environmental and occupational exposures, as well as chemicals in foods, consumer products, and over-the-counter medications.
We are recognized for tackling society’s most complex challenges, including assessing cumulative health risks for vulnerable populations and utilizing new approach methods (NAMs) to reduce reliance on animal models in research and human health risk assessment applications. Our team has world-renowned expertise in conducting systematic literature reviews and evidence-based toxicology.
BlueRidge employs physiologically based pharmacokinetic (PBPK) models and advanced in silico tools for modeling metabolism and quantitative structure-activity relationships (QSAR), refining risk assessment methods to enhance decision-making and address regulatory and legal challenges. Additionally, we conduct dose-response modeling, mode-of-action analyses, and adverse outcome pathway studies while designing and overseeing research that supports safety mechanisms of action.
Food and Consumer Product Safety
BlueRidge toxicologists and regulatory scientists assist food and consumer product companies in ensuring safety, maintaining compliance, and substantiating claims, including those related to sustainability.
We conduct comprehensive safety and risk assessments for foods, dietary supplements, cosmetics, personal care products, and raw ingredients, including complex botanicals and mixtures. Our team has deep expertise in regulatory expectations, including the Food Safety Modernization Act (FSMA) and the Modernization of Cosmetics and Regulation Act (MOCRA). Additionally, we provide support in landscape and horizon scanning topics and offer extensive experience in food safety plans, specification considerations, food defense risks, and validation assistance for food processing operations.

SECTORS WE SERVE
BlueRidge continues to drive innovation on behalf of industry leaders in pharmaceutical, food, consumer product, chemical, energy, manufacturing, and other businesses.
-
Developers of innovative drug/biologic + device products turn to BlueRidge for support from planning through commercialization, reducing costs and increasing revenue.
-
Makers of ingredients, food products, and dietary supplements rely on BlueRidge to assess and ensure safety, substantiate health claims, and evaluate regulatory compliance risks.
-
State and federal agencies select BlueRidge for our deep scientific expertise supporting safety assessments in pharmaceutical products and for the study of challenging environmental issues related to endocrine disruption and environmental exposure to chemicals.
-
A broad spectrum of leading companies in various industries, regional entities, and trade associations partner with BlueRidge for scientific services that address human and environmental health concerns.
-
Attorneys requiring scientific expertise to understand exposure, toxicology, and health risk tap BlueRidge scientists to conduct in-depth research into causation analysis.
-
Leading consumer product companies trust BlueRidge toxicologists to conduct safety and risk assessments that satisfy regulatory requirements on existing and newly developed products.
-
Animal feed and pet food companies turn to our toxicologists for comprehensive safety assessments, regulatory strategies, and assistance with product claims substantiation.
-
Pharmaceutical and biotech companies choose BlueRidge to support their pre-clinical and clinical development, distribution, regulatory and commercial activities to seamlessly get new and life-changing interventions to those who need them.
-
Academic institutions and organizations partner with BlueRidge to strengthen study design, enhance research methodologies, and garner regulatory approval to translate new discoveries into meaningful health solutions.
INDUSTRY IMPACT
Our highly experienced world-renowned scientists, physicians, and engineers, help premier companies get further faster, bringing their life science goals to fruition.